Wellness Insights
- Home
- Wellness Insights
- Oral Bioavailability Is the Key to the Efficacy of Silybin (Silymarin)
2022.03.25
Wellness InsightsOral Bioavailability Is the Key to the Efficacy of Silymarin
Pharmacist Kuo, Kuo-Hua (Former Professor, Kaohsiung Medical University)
Silymarin is extracted from the Milk Thistle plant, which originates from the Mediterranean region. As early as ancient Greece and the early centuries of Europe, records show the use of Milk Thistle to treat diseases and reduce jaundice. Therefore, the use of Milk Thistle for liver protection in Europe has a history of several thousand years.
Today, silymarin is globally recognized in academia as a liver-protective ingredient. More than 4,000 international academic papers have confirmed it as one of the safest and most effective hepatoprotective compounds. Relevant products can be found in pharmacies, drugstores, and health food markets around the world.
Silymarin was first developed and certified as a pharmaceutical by a German pharmaceutical company. It was registered and approved in Taiwan and China decades ago as an “advanced pharmaceutical product,” and therefore only Germany, Taiwan, and Mainland China currently classify silymarin as a regulated drug.
Other countries such as the United States, Japan, South Korea, Australia, New Zealand, and Southeast Asia sell silymarin as a food supplement. Although the U.S. has included silymarin in its pharmacopeia, it is not approved as a therapeutic drug because common commercial products exhibit “low oral absorption (low bioavailability),” resulting in unclear efficacy.
Therefore, researchers worldwide have been striving to overcome the bottleneck of “low bioavailability.” Numerous studies are published every year proposing technologies, methods, and formulations to improve absorption.
Pharmacopeias around the world regulate pharmaceutical quality and testing standards. Both the United States Pharmacopeia and Taiwan’s 8th Edition Chinese Pharmacopoeia stipulate that drugs must have adequate dissolution rates to be absorbed by the human body. According to pharmacopeial testing methods, silymarin products must achieve a dissolution rate of greater than 75% to meet the standard.
After collecting and analyzing commercial silymarin products from the United States, Germany, Japan, Taiwan, and Singapore, the comparison results based on the U.S. Pharmacopeia are shown below:
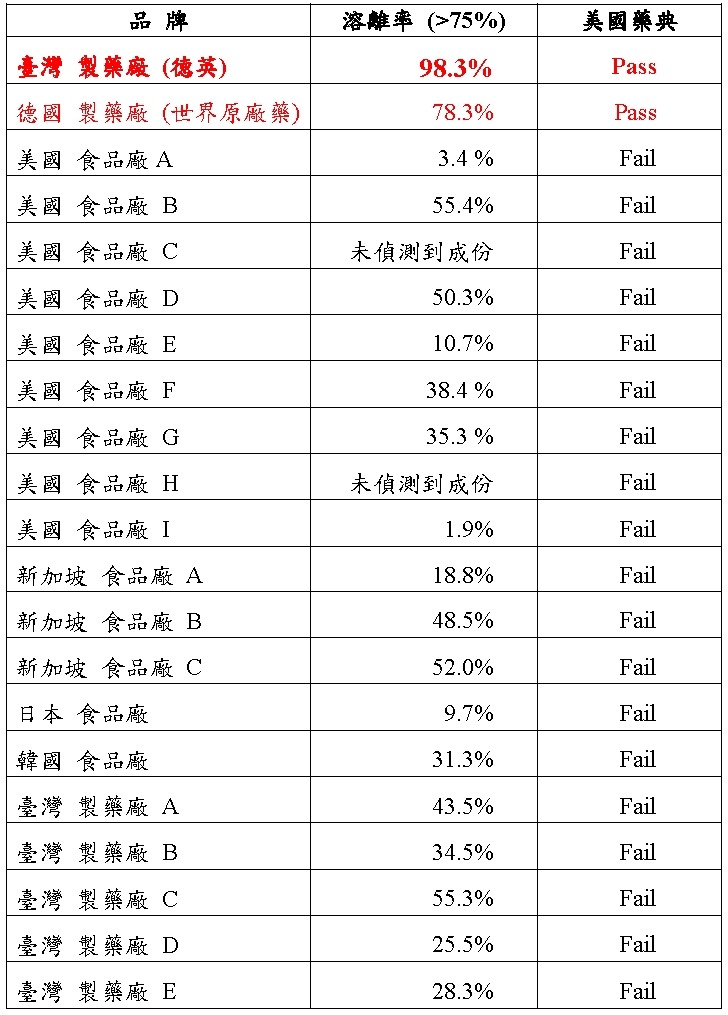
According to the U.S. Pharmacopeia analysis, only the German original pharmaceutical product and Taiwan’s DEING Pharmaceutical silymarin liver medication fully meet pharmacopeial testing standards.
Among them, the silymarin liver product developed by DEING Pharmaceutical Taiwan demonstrates the highest bioavailability among global products of the same ingredient, and fully meets the standards of both the United States Pharmacopeia and the Chinese Pharmacopoeia.
DEING Bio continues to uphold scientific research as its foundation and is committed to developing high-quality, highly absorbable liver-protective medications to safeguard public health.
